WHO Approves Emergency Use of Monkeypox Diagnostic Test
Geneva — the World Health Organization (WHO) announced the emergency approval of a diagnostic test for mpox (monkeypox), marking a crucial milestone in expanding global access to monkeypox testing.
Geneva, Oct. 4 (Xinhua) — On Friday, the World Health Organization (WHO) announced the emergency approval of a diagnostic test for mpox (monkeypox), marking a crucial milestone in expanding global access to monkeypox testing.
The approval comes as Africa continues to face challenges due to limited testing capacity, which has allowed the virus to spread rapidly. Africa has already reported more than 30,000 suspected cases in 2024.
The WHO stated that the Democratic Republic of Congo (DRC), one of the three countries most affected by mpox alongside Burundi and Nigeria, tested only 37% of suspected cases in 2024.
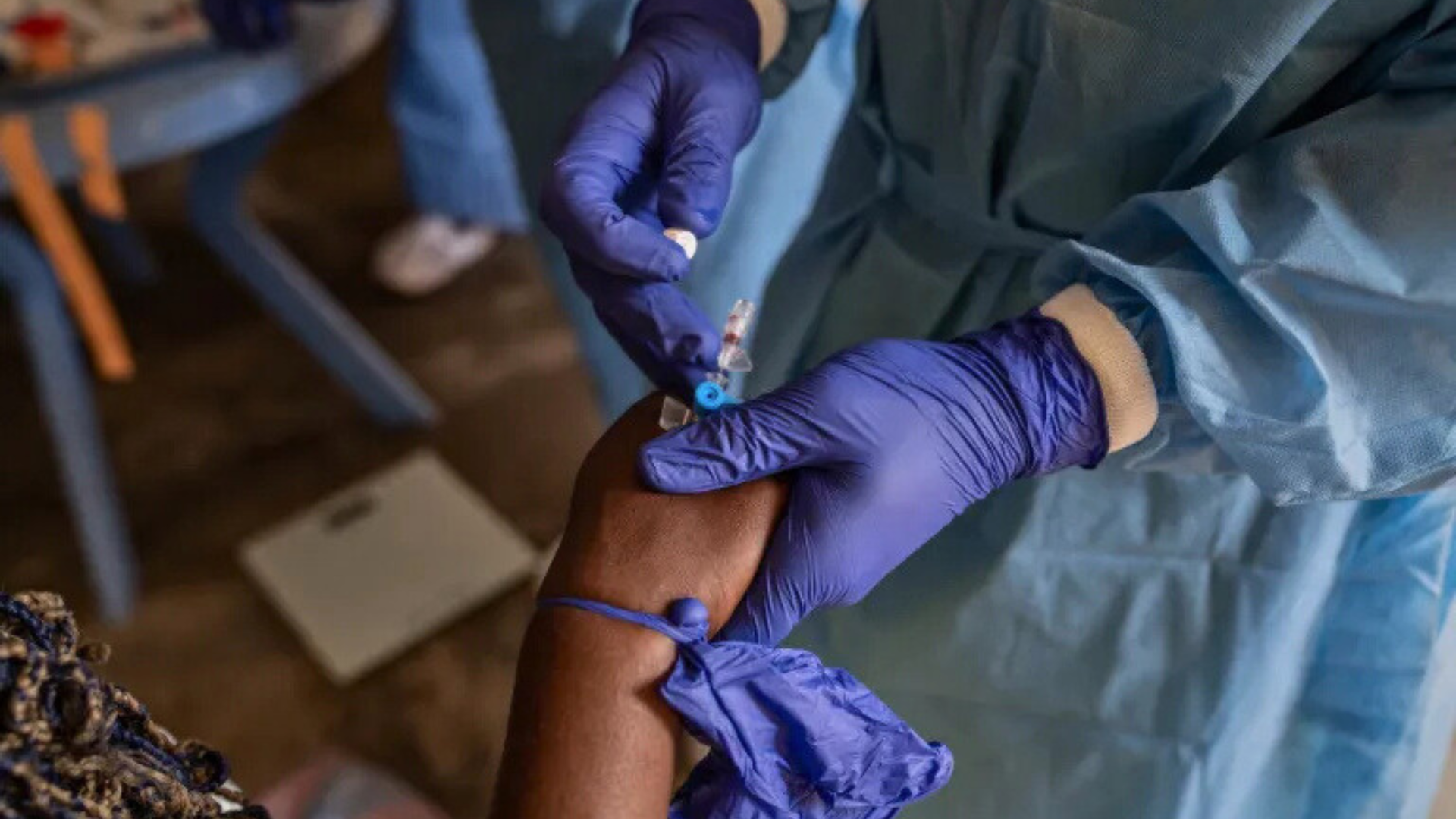
The approved diagnostic test is expected to enhance testing capacity in several countries facing mpox outbreaks. Rapid and accurate diagnosis is key to controlling the spread of the virus.
The approved test, called Alinity m MPXV, is a real-time PCR test designed to detect the mpox virus's DNA through swabs taken from skin lesions. It allows laboratories and health professionals to confirm the disease quickly.
Yukiko Nakatani, WHO’s Assistant Director-General for Access to Medicines and Health Products, emphasized that the emergency use listing of the monkeypox diagnostic test represents a significant step in increasing testing accessibility for affected countries.
Sources:
此类别中的文章由我们的编辑团队撰写,旨在让您了解最新的医疗保健和医疗旅游新闻。
更多新闻

Dr. Korravee Tangekachai from Seoul Clinic Participates in the Deep Plane Facelift & Rhinoplasty Congress to Exchange Knowledge and Update on the Latest Surgery Trends
October 9, 2024

BDMS Wellness Clinic Teams Up with Sri Panwa Phuket and Bangkok Hospital Phuket to Create Thailand’s First Six-Star Scientific Wellness Experience
October 10, 2024